Dupuytren's Recurrence
A
personal account of problems encountered
with Dupuytren's surgery and an
examination of the causes and avoidance
of subsequent recontracture
INTRODUCTION
I suffer from Dupuytren's contracture. It's a condition that afflicts mostly men in late middle age and results in their being unable to fully extend one or more of their fingers. The problem can be corrected with a simple operation but, despite the fact that all the material that needs to be excised lies no more than a few millimetres below the surface of the skin and its removal should be straightforward, patients can have very different experiences both in the short term recuperation phase and with long-term recontracture. In the following text, I will first describe the very different experiences I have had with my two hands. I will then cover some basic anatomy and follow this with a look at the histological reasons for recontracture. I will then identify one procedure that does give good results and which every sufferer of this complaint should know about. In creating this web site, I hope to aid others with Dupuytren's by helping them to avoid some of the problems that I have encountered.
Let me say at the outset, and make it clear, that I consider that I have been unfortunate to have encountered one surgeon who was plainly incompetent. I make no criticism of the large majority of surgeons who are conscientious and dedicated to the welfare of their patients. That being said, the appalling figures for the rate of recurrence of this complaint are not the result of the failings of just one surgeon.
MY EXPERIENCE
My right hand was the first to be affected and it was operated on 1997 with reasonable success. I didn't realize it at the time but I was lucky to be operated on by a surgeon who was interested in developing less invasive techniques and was, as a result, achieving much higher success rates.
In 2005, my left hand also became affected. Unfortunately, the surgeon who operated on me previously was not available and I was persuaded to go to another who, it was claimed, specialized in Dupuytren's. The fingers affected and their degree of contracture were identical to the condition of my right hand when it was operated on. So, not unreasonably, I assumed that this specialist would do at least as good a job as the first surgeon. However, five years after the operation, my left hand is now far worse than the condition it was in before surgery. In fact, it is so bad that further corrective surgery is unlikely to be successful.
My Right Hand
Fig 1, on the left, shows my right hand just
prior to the operation. Note that the main contracture was in the
fourth and fifth fingers. There was also partial contracture of the
third finger. (It is the custom in the medical profession to identify fingers numerically 1 to 5 starting with the
thumb and ending with the little finger).

 The picture on the right, fig 2, was taken about one week after the operation.
The surgeon told me that he had not found the use of a splint (or cast) to
be beneficial, so, he left the hand loosely wrapped in a bandage which
allowed me free movement of my fingers. This encouraged blood
flow which avoided clots and greatly accelerated the healing process. Another contributory
factor to the healing was the perfect closure. In fact, I was
very surprised that, apart from the dried blood visible along the line of
the incision, there was no bleeding. Also, throughout the healing
process, I lost no feeling or strength in my fingers. Looking
back, I can honestly say that the recuperation phase hardly impacted on my
life at all. When the time came to remove the stitches, about
half of them had already fallen out because the healing process was so far
advanced.
The picture on the right, fig 2, was taken about one week after the operation.
The surgeon told me that he had not found the use of a splint (or cast) to
be beneficial, so, he left the hand loosely wrapped in a bandage which
allowed me free movement of my fingers. This encouraged blood
flow which avoided clots and greatly accelerated the healing process. Another contributory
factor to the healing was the perfect closure. In fact, I was
very surprised that, apart from the dried blood visible along the line of
the incision, there was no bleeding. Also, throughout the healing
process, I lost no feeling or strength in my fingers. Looking
back, I can honestly say that the recuperation phase hardly impacted on my
life at all. When the time came to remove the stitches, about
half of them had already fallen out because the healing process was so far
advanced.
My Left Hand
The surgeon who operated on my left hand showed no interest in the work done on my other hand. In fact, on each of three occasions when I questioned the necessity for a splint, he immediately changed the subject. He did believe in the beneficial use of a splint, no matter what the evidence to the contrary. So, when I came round from the anaesthetic, I found my left hand encased in a crude but very effective cast that held my fingers rigidly and fully extended. Another distinctive feature of the cast was that it was saturated with my blood. When I pointed out that I would have to keep the whole thing in a plastic bag to prevent the blood getting on my clothing, my protests were dismissed - indeed, it was all treated as a huge joke. However, it is noteworthy that, when I was leaving, a male nurse waylaid me and wrapped a bandage around the offending appendage, not to protect my clothing but rather to "...avoid frightening the people in the waiting room". Needless to say, wearing this cast for the next two weeks was no picnic; not just because of the inconvenience but also because it stank of stale blood every time it got damp. However, that was nothing compared with the shock I got when it was removed.
 The
first things I saw were three long zigzag incisions - not the single 8cm
incision used by the first surgeon. In fact, the total length of these
three incisions was a staggering 37cm (Fig 3). A separate incision had
been made for each of the fourth and fifth fingers instead of the single
incision used by the first surgeon. Also, he had operated on the third
finger which was completely unnecessary. I'll
explain the reason for this later. For the moment, note that the third
finger of my right hand (see Fig 1) appears to be contracted. However, after the
operation, it was straight despite the self evident fact that the surgeon
had not operated on it (see Fig 2).
The
first things I saw were three long zigzag incisions - not the single 8cm
incision used by the first surgeon. In fact, the total length of these
three incisions was a staggering 37cm (Fig 3). A separate incision had
been made for each of the fourth and fifth fingers instead of the single
incision used by the first surgeon. Also, he had operated on the third
finger which was completely unnecessary. I'll
explain the reason for this later. For the moment, note that the third
finger of my right hand (see Fig 1) appears to be contracted. However, after the
operation, it was straight despite the self evident fact that the surgeon
had not operated on it (see Fig 2).
We then came to the business of removing the stitches. This would not have been such a problem if everything had not been caked with dried blood. I presume, in an attempt to lighten the increasingly tense atmosphere, the surgeon quipped that he would have to be careful as, in the past, he had been told off for not removing all the stitches. Watching him poking around in the mess that covered the palm of my hand, I did not find that at all surprising. I was, however, surprised that I could not feel the stitches being removed. Although the whole process with my right hand had been pain free, the one procedure that was quite painful was the removal of the remaining stitches. This was to be expected as I had never lost sensation in that hand during the healing process. I was now becoming very concerned that I couldn't feel anything over the palm of my left hand or up its fingers. I mentioned this at the time and on several occasions thereafter until, eventually, the surgeon gave the ill-tempered response, "of course your hand is going to be numb". In fact, there is absolutely no inevitability about this. Moreover, it is an ominous indication of trouble to come. Assuming no nerve damage, a lack of sensation is probably due to local ischaemia (inadequate blood flow), a condition that promotes fibroblast proliferation. I shall explain how this happens and the consequences later. Meanwhile, about three weeks after the stitches were removed, I found a stitch that the surgeon had missed. He had cut it off flush with the surface of the skin - the one thing that should be avoided.
In addition to the lack of sensation, I was quite unable to move my fingers. Their enclosure in the splint left them jammed in their fully extended position and nothing I could do would move them. This situation persisted for two or three days until, following constant manipulation, I was able to move them a little. It was seven months before I could touch the palm of my hand with the tips of the fingers. Over this period, there was also an almost complete loss of strength in the fingers. This condition slowly ameliorated over the following two years but, five years after the operation, I still have not regained the former strength in these fingers.
I have said that the first surgeon, who operated on my right hand, had closed the incision perfectly and that the healing was rapid. Unfortunately, the same cannot be said of the second surgeon. I think that I can reasonably claim that I have a strong stomach. I am not sickened by the sight of blood or injury. Certainly, when the splint was removed, it was not the sight of the congealed blood that coated my hand that disturbed me. What really appalled and nauseated me was the crude, amateurish closure and the fact that I could see very little evidence of healing. Instead of close and neat abutment of the edges of the incisions, there were numerous deep fissures and rucks (places where, between two stitches, one side of the incision lays flat whilst the other side is rucked up). Considering that, over the same period since the operation, the incision on my right hand had healed over, it was appalling that my left hand looked as if the operation had only just concluded. Later, after I had arrived home, my fears were borne out as all the wounds started to open up and suppurate. Unable to get help, I was obliged to try to hold the wounds together with strips of adhesive tape whilst a pad of lint retained the constant discharge of puss and blood. Adhesive straps were still in place when I returned to the surgeon about two weeks later. He made no comment about them and stated only, "Oh, that's fine". Now, it seems to me that there's nothing 'fine' about this and most professional surgeons would not think it 'fine' that their patients had to provide their own closure.
In view of the ample opportunity for infection during this early phase, I was paying particular attention for any signs of this. As the wounds finally started to heal over, I began to think that I was over the worst when, slowly, the whole hand started to swell up. At its worst, I could not move my fingers at all and, for about three weeks, I had to endure a constant throbbing pain until the swelling eventually abated.
The surgeon had always maintained that these small problems would be solved with plenty of vigorous massage and exercise. So, as soon as conditions allowed, he arranged for me to attend the physiotherapy department at my local community hospital. He said something to the effect that it would be all right as he had spoken with them. When I arrived, I was met by a young chap who seemed strangely reluctant to look at my hand. Instead, we just talked at length about the subject. When, eventually, I asked if he was going to start the treatment, he called his supervisor. This obviously irritated individual came in and immediately told me that the surgeon had visited their department to explain that the success rates for this operation were always going to be poor and, consequently, there was nothing that they could (or would) do. With that, the two of them turned and walked out of the room. Neither of them made any attempt to examine my hand. When I related these events to the surgeon, he was plainly annoyed, not that I had failed to received the treatment I needed but because he had failed to persuade that physiotherapy department to treat his patients. One can only surmise as to the full reasons for the physiotherapist's refusal to treat me but it was made very clear to me that they had made the decision not to treat the patients of that particular surgeon and they were not going to change their minds. Considering that they must have come to their decision after having had patients referred to them by that surgeon in the past, one wonders how long this has been going on and how many patients had been refused treatment? Also, just how bad does a surgeon have to be for a physiotherapy department to refuse to treat his patients. It is revealing to consider that all this was going on under the noses of National Health Service (NHS) executives, some of whom must have been involved as the people I met were unlikely to have acted in the way that they did without first getting the consent of their senior manager. One wonders just how many of the NHS management knew that there was a problem with that surgeon. I think someone should ask them why it was that the only action they took was to deprive their vulnerable patients of essential physiotherapy rather than address the actual cause of the problem.
There was nothing unique about my case. Mine was the most common form of Dupuytren's contracture. As I have already stated, the same fingers on both hands were contracted to the same degree. So, why is it that, while one surgeon can clearly demonstrate that it is possible to perform this operation with minimal recurrence, another surgeon, working on the same condition in the same hospital, is allowed to produce such grossly inferior work and continue to do so with impunity? Why is it that we patients are subject to this grotesque lottery?
Long Term Results
I stated in the introduction that the long term success rate
for this operation is very poor. Taking some published figures[15],
the following table gives a rough guide to the rates of recurrence:
| Years after Operation | Recurrence |
| 3 | 48% |
| 5 | 62% |
| 10 | 70% |
The figures in this table are also in close agreement with other surveys that I have seen. However, I suspect that the actual number of recurrences are even worse than these published figures. The definition of recurrence is usually taken to mean the reappearance of Dupuytren's tissue in the zone previously operated on. It does not include lesions that appear in adjacent and previously unaffected joints. So, according to that criterion, the operation on my left hand (Fig. 4) has been a complete success because the MP joints are still straight. The condition of the PIP joints is ignored even though their present contracture was caused by the excessive trauma of the operation on the MP joints. This clearly demonstrates how misleading these figures for recurrence can be. I also have to say that some surgeons are happy for this misunderstanding to continue. One such asked me, "What are you complaining about? The MP joints are straight, aren't they?" If the contracture of the PIP joints is the price to be paid for straightening the MP joints then the operation cannot in any sense be regarded as a success.
Also, considering that recovery takes most of the period leading to the point when another operation would become necessary, the patient can find himself faced with the prospect of endlessly recuperating from botched operations and an ever diminishing likelihood of success. However, if, as with my right hand, the surgeon makes every effort to minimize the trauma, subsequent contracture, especially in adjacent joints, can be minimized. The following images illustrate the point.


The picture on the right was taken 13 years after the operation on my right hand. Although some recontracture has occurred, particularly in the PIP joint of the fifth (small) finger, at least it's not getting worse. Recurrence in the little finger is particularly difficult to avoid because of the small scale and unique ligament attachments that I will describe later. The picture on the left was taken just four years after the operation. Recontracture is severe and getting worse. It is worth reiterating that both my hands were in the same condition before they were operated on. That is to say, the same fingers were contracted to the same degree. The only difference was that they were operated on by different surgeons who used very different procedures.
Now, in order to understand why these procedures have such an influence on the outcome, I need to explain a little basic anatomy.
ANATOMY
A Few Terms and Definitions
The terms Proximal and Distal are widely used in anatomical reference. Proximal means 'nearer' or 'closer to some point or median line' and Distal means 'further' or 'furthest from some point or median line'.
Each of the three finger (or toe) bones is called a Phalanx (plural: Phalanges. Not Phalanxes: bodies of troops or police etc.). Of the three bones in each finger, the one nearest the palm is the Proximal Phalanx, then comes the Middle Phalanx and finally the Distal Phalanx. The thumb has just two Phalanges. The five Metacarpal bones lie between the fingers (and thumb) and the Carpus bones in the wrist.
Joints are identified as follows:
MP - metacarpophalangeal (Joint between metacarpal and proximal phalanx)
PIP - proximal inter-phalangeal (Nearest joint between two phalanx bones)
DIP - distal inter-phalangeal (Distant joint between two phalanx
bones)
Collagen is a protein that is the
principal constituent of white fibrous connective tissue. It is found
in ligaments, tendons, cartilage, skin and bone. It is flexible with high
tensile strength.
Please Note: in the following text, I
shall use the term 'diseased' to refer to contracted cords made of collagen.
It is a term widely used in medical circles when referring to tissue that is
causing contraction which has to be excised to achieve extension of the
fingers. However, as I shall describe later, the actual contractile
tissue is not those bands of collagen that form part of the normal anatomy
of the hand but rather fibrous material that has been deposited on them.
I have seen no convincing evidence that the original anatomical bands
of collagen
become 'diseased' in the true meaning of that word.
Fibrous Skeleton - In addition to the osseous (bony) skeleton, there is a skeleton of collagenous (made of collagen) fibres. It can be thought of as a flexible scaffolding that holds all the components of the hand, including the skin, in relation to one another whilst allowing considerable flexibility. The central region of the hand has three layers of these fibres: the Superficial Palmar Fascia which lies just beneath the skin of the palm, the Deep Palmar Fascia and the Dorsal Fascia which is on the back of the hand. It is generally the case that only the first of these, usually simply referred to as the Palma Fascia, is subject to Dupuytren's disease. In addition, there are groups of fibres that pass between each of these layers. The overall structure serves to hold the various components of the hand separate and in position when it is subject to the stresses of normal usage.
In the following diagrams, the main arteries are shown in red and the main nerves are shown in yellow. They tend to be grouped together in pairs called Neurovascular Bundles.
The Hand
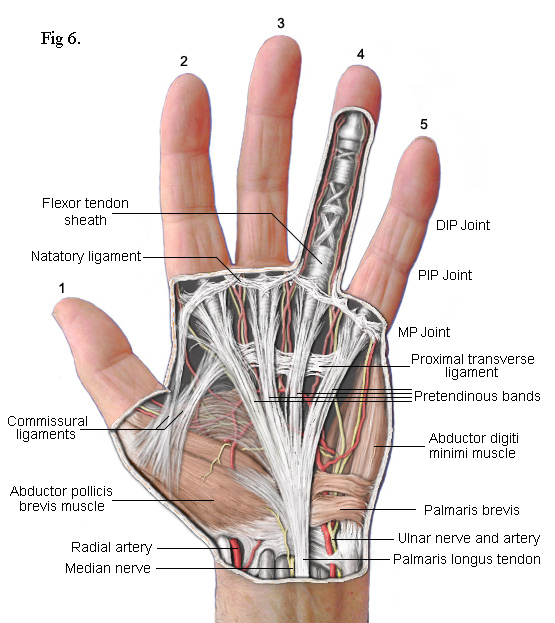 The
drawing on the left shows the principal subcutaneous features. False colour
is used to enhance clarity. The main feature is the part of the palmar
fascia which consists of a series of fibres that pass up from the
wrist and spread out, fan like, across the palm. These fibres bunch
together into four groups called the Pretendinous bands. Each band is
aligned with one of the four fingers. As each bunch approaches the base of
its respective finger, clusters of fibres break away to form separate
connections. Most pass upwards to the skin where they form the
cutaneous creases of the palm. Others pass down to a deep transverse
metacarpal ligament (not shown) whilst others pass into the finger on either
side of a central Flexor Tendon Sheath. Contracture of the MP joints
is caused by Dupuytren's disease in
these pretendinous bands.
The
drawing on the left shows the principal subcutaneous features. False colour
is used to enhance clarity. The main feature is the part of the palmar
fascia which consists of a series of fibres that pass up from the
wrist and spread out, fan like, across the palm. These fibres bunch
together into four groups called the Pretendinous bands. Each band is
aligned with one of the four fingers. As each bunch approaches the base of
its respective finger, clusters of fibres break away to form separate
connections. Most pass upwards to the skin where they form the
cutaneous creases of the palm. Others pass down to a deep transverse
metacarpal ligament (not shown) whilst others pass into the finger on either
side of a central Flexor Tendon Sheath. Contracture of the MP joints
is caused by Dupuytren's disease in
these pretendinous bands.
The palmar fascia also includes several collagenous transverse ligaments. Normally, these do not become
diseased but one that does is the Natatory Ligament (from 'schwimmband' by Braune[2]).
This ligament passes across the palm at the base of the fingers. Fibres
within it pass from the Flexor Tendon Sheath of each finger into the lateral
space of the adjacent fingers extending as far as the distal phalanx.
When this ligament becomes diseased and contracts, it causes the fingers to
be drawn together (see fingers 4 & 5 in Fig 1). It can also transmit
stress from a diseased pretendinous cord of one finger to an adjacent finger. Thus, whilst a
pretendinous cord will cause its respective finger to contract, the natatory
ligament can also cause an adjacent finger to partially
contract without there being any disease in its pretendinous band. The third finger of my right hand was affected in this way
(Fig 1). When the surgeon released the pretendinous cord of its fourth
finger, the third finger was also released. That, and the fact that I
couldn't feel any pretendinous cord to the third finger, is how I know that the
third finger of my left hand did not need to be operated on.
Note: The terms 'band' and 'cord' have specific meanings. In 1959, Luck[12] established the convention whereby normal fibrous tissue is referred to as 'band' and diseased tissue as 'cord'.
 Normally,
the thin strands of collagen that make up the main components of the palmar
fascia are separate. However, Dupuytren's
disease causes these strands to become enveloped by
new collagen into a single, much thicker, cord. So, numerous individual
strands in a pretendinous band become a single thick cord. Within this
cord are two distinct arrangements of fibrils. The linear form has fibrils
arranged in densely compacted straight lines extending longitudinally along the cord
and, embedded
within it and superficially, there are small 'nodules' made of randomly arranged fibrils
interspersed with cellular tissue. These give the
cord a 'lumpy' appearance.
Normally,
the thin strands of collagen that make up the main components of the palmar
fascia are separate. However, Dupuytren's
disease causes these strands to become enveloped by
new collagen into a single, much thicker, cord. So, numerous individual
strands in a pretendinous band become a single thick cord. Within this
cord are two distinct arrangements of fibrils. The linear form has fibrils
arranged in densely compacted straight lines extending longitudinally along the cord
and, embedded
within it and superficially, there are small 'nodules' made of randomly arranged fibrils
interspersed with cellular tissue. These give the
cord a 'lumpy' appearance.
Central Cord - In a high proportion of cases, the pretendinous cord will follow a midline course and attach itself via a large nodule to the tendon sheath of the proximal phalanx (Fig 7). Thus, contraction of the pretendinous cord prevents the proximal phalanx from fully extending. In some cases, this cord will extend further along the midline to the middle phalanx attaching itself to its tendon sheath and contracting the PIP joint. Many strands of the normal pretendinous band pass upwards to the cutaneous layers where they are responsible for the creases in the palm. So, when this band becomes infected, deep folds and indentations develop in the skin. At the same time, the contracting cord becomes substantially thicker and forms a prominent palmer ridge in line with the finger (Fig 1).
As the cord is mostly superficial, its surgical extraction (fasciotomy) is fairly simple. However, the developing cord also invades the subdermal layers along its entire length and has to be carefully separated. This inevitably results in some loss of the fatty subdermis which risks compromising the viability of the skin. The neurovascular bundles are relatively unaffected but, when the central cord combines with a lateral cord (a diseased lateral sheet), the neurovascular bundle can be displaced towards the midline putting it at risk of damage during surgery. There are numerous ways in which the pretendinous band, the natatory ligament, the lateral sheet and other ligaments in the finger can become individually or collectively diseased. The most common combinations of these are depicted in the following simplified diagrams. Figs 8 and 9 each contrast the unaffected lateral sheet on the left with the affected lateral cord on the right.
The Lateral Cord in Fig 8 proximally adheres to the natatory cord which also contracts preventing the adjacent finger from separating. The exception is the little finger where the natatory cord is also attached to the tendon of the abductor digiti minimi muscle (Fig 10). Another ligament to be involved is the Grayson's ligament[9] which, together with the Cleland's ligament (deeper and not shown), provides a conduit for the neurovascular bundle, securing it when a finger flexes. Cleland's ligament is much thicker and not usually affected by Dupuytren's disease but Grayson's ligament becomes enveloped by the spreading collagen and included into the lateral cord. So, as Grayson's ligament is attached to the tendon sheath at the base of the middle phalanx, when the whole cord contracts, it closes the finger at its PIP joint. This cord may also extend to the distal phalanx causing contracture of the DIP joint. Because the lateral sheet is normally inserted into the skin along most of its length, the lateral cord becomes deeply attached making it difficult to remove without damaging the subdermal layers.


The Spiral Cord is similar to the lateral cord but differs in its proximal attachment. In the case shown in Fig 9, the attachment is to the pretendinous cord. Unlike the lateral cord, the spiral cord passes under the neurovascular bundle at the base of the finger and, as the cord straightens, this bundle is forced to spiral around it. As can be seen, the neurovascular bundles are displaced by both the lateral and spiral cords. In the latter case, the bundle is also pushed superficially making it particularly vulnerable to damage during surgery.
 There
are also Retrovascular Bands that can become diseased. I've not
shown them in the above sketches partly because they are located behind the
neurovascular bundles - hence their name. Like the spiral cords, they
originate from the pretendinous cords. Passing laterally up the finger,
they form connections with the natatory ligament, the proximal phalanx, the
proximal end of the middle phalanx and end up bonded intimately to the collateral
ligaments of the DIP joint. However, on the ulna side (on the same side as the ulna bone in
the forearm - i.e. opposite the thumb) of the little finger, the retrovascular cord is almost always connected to the tendon of the abductor digiti minimi
(ADM)
muscle - see Fig 10. As stated earlier, the little finger commonly
suffers contracture resulting from attachment of the natatory ligament to
the tendon of this muscle. The combined affect
is to splay the finger laterally and rotate it at the same time. Added
to this, as the disease invariably spreads into the retrovascular band, all
the joints in the little finger can become contracted as well.
There
are also Retrovascular Bands that can become diseased. I've not
shown them in the above sketches partly because they are located behind the
neurovascular bundles - hence their name. Like the spiral cords, they
originate from the pretendinous cords. Passing laterally up the finger,
they form connections with the natatory ligament, the proximal phalanx, the
proximal end of the middle phalanx and end up bonded intimately to the collateral
ligaments of the DIP joint. However, on the ulna side (on the same side as the ulna bone in
the forearm - i.e. opposite the thumb) of the little finger, the retrovascular cord is almost always connected to the tendon of the abductor digiti minimi
(ADM)
muscle - see Fig 10. As stated earlier, the little finger commonly
suffers contracture resulting from attachment of the natatory ligament to
the tendon of this muscle. The combined affect
is to splay the finger laterally and rotate it at the same time. Added
to this, as the disease invariably spreads into the retrovascular band, all
the joints in the little finger can become contracted as well.
If the lateral cord is also present, it too becomes attached to the tendon of the ADM muscle.
There is a lot of variability between patients. The spiral cord, for example, may not originate from the pretendinous cord but from fibres at a deeper level and spiral cords may combine with long central cords contracting MP and PIP joints. The number, arrangement and termination of the pretendinous cords varies considerably. Within the fingers, any combination of central, lateral, spiral and retrovascular cords can also exist to any degree of involvement.
Now, it is a surgical priority to quickly and safely locate the neurovascular bundles so as not to accidently damage them. To this end, there have developed over the years a wide variety of incisions that variously claim not only to facilitate this but also to give adequate exposure of the diseased tissue, reduce skin necrosis, avoid contractile scarring and so on. Apart from the first surgeon who operated on my right hand, all surgeons with whom I have spoken proposed to use the zigzag type Bruner[4] incision. This was first published back in 1951 but has retained its popularity with plastic surgeons as it allows skin lengthening and improves access. However, skin lengthening was certainly not a requirement in my case, partly because I had not left it too long before consulting a surgeon. Also, extending separate incisions from each finger into the palm creates a problem. The centre of the palm is poorly vascularized[5,15] (i.e. there is a paucity of arterial blood supply) resulting in a risk of skin necrosis at the tips and exacerbating a problem with blood clot dispersal which I shall describe more fully later. In other words, habitually applying some standard incision cannot possibly be in the patients best interests. It should go without saying that the incision should be minimal and adapted to the clinical conditions.
Also, before we leave the subject of anatomy, it is worth mentioning an interesting feature of the digital web space that can facilitate the quick and safe location of the neurovascular bundles.
 The web space is the soft tissue between the bases of the fingers (and
toes). Some of the fibres of the pretendinous band, that pass either
side of a flexor tendon sheath, pass below the neurovascular bundles and
form a coalescence of fibres attached to the subdermal tissue directly
between two fingers. See Fig 11. Immediately distal from this point and on either side of it
(indicated by the two arrows), the
skin does not adhere to the underlying fibrous tissue. Making an
opening incision at this point enables the surgeon to quickly locate the
neurovascular bundle and the fibrous cords that need to be removed.
Now, if we look again at the incision used by the first surgeon on my right
hand, Fig 2, or the tracing of it at Fig 12, we can see the very significant fact that the incision goes
directly over these two points. The place where the opening incision
would have been made is shown in blue. Of course, I can't be absolutely sure
that he was making use of this anatomical anomaly because I didn't think to
ask him at the time. However, I now think it highly likely that he
did. This type of incision gives good access,
offers good insurance against accidentally damaging the neurovascular
bundles, is far less invasive and is much more professional than to mindlessly repeat
the Bruner incision irrespective of the clinical conditions.
The web space is the soft tissue between the bases of the fingers (and
toes). Some of the fibres of the pretendinous band, that pass either
side of a flexor tendon sheath, pass below the neurovascular bundles and
form a coalescence of fibres attached to the subdermal tissue directly
between two fingers. See Fig 11. Immediately distal from this point and on either side of it
(indicated by the two arrows), the
skin does not adhere to the underlying fibrous tissue. Making an
opening incision at this point enables the surgeon to quickly locate the
neurovascular bundle and the fibrous cords that need to be removed.
Now, if we look again at the incision used by the first surgeon on my right
hand, Fig 2, or the tracing of it at Fig 12, we can see the very significant fact that the incision goes
directly over these two points. The place where the opening incision
would have been made is shown in blue. Of course, I can't be absolutely sure
that he was making use of this anatomical anomaly because I didn't think to
ask him at the time. However, I now think it highly likely that he
did. This type of incision gives good access,
offers good insurance against accidentally damaging the neurovascular
bundles, is far less invasive and is much more professional than to mindlessly repeat
the Bruner incision irrespective of the clinical conditions.
HISTOLOGY
Histology is the study of the microscopic structure of tissues - a subject in which I have a particular interest. As we have seen, collagen is a white, inelastic, fibrous constituent of 'connective tissue'. Connective tissue acts as a packing tissue or serves to separate organs. It consists of an amorphous 'ground substance' which can also contain elastic and reticular fibres (microscopic branching fibres that join together to form a supportive mesh for blood vessels, muscle fibres etc.), fat cells, mast cells (large cells that are a source of histamine, serotonin and heparin), macrophages (scavenger cells) and fibroblasts. Variations in the proportions of these constituents gives rise to the widely differing characteristics of connective tissue found throughout the body.
Collagen - Collagen is a major constituent of the body. It accounts for about 30% of the protein mass in mammals and is found in skin, bone, tendons, cartilage - even in the lens of the eye. It is divided into molecular types of which some 28 have been described in the technical press. Collagen found in connective tissue is usually of type I, II, III, V or XI. Dupuytren's contracture is mainly linked with just two of these - types I and III. Type I is the most abundant, accounting for 90% of all collagen in the body. It is very resistant to tensile (stretching) force. Type III gives structural support and is somewhat more elastic than type I. Importantly for this text, it is the principal constituent of granulation tissue which is created as part of the normal healing process.
Collagen does not have a cellular structure. It is protein. That is to say, it is a complex structure composed of many chains of amino acids, linked by peptide bonds. Typically, the basic component of collagen is composed of three strands of helical polypeptide chains wound around one another to form a triple-helical structure. When the body needs to create collagen - for example, as part of the healing process - it synthesizes these long molecules in cells called fibroblasts which then secrete and align these chains side by side to form fibrils of about 0.01 to 0.3 micron diameter (a 'micron' is one thousandth of a millimetre). The fibroblast can then arrange these fibrils and, given the right conditions, it will align them with other fibrils to create strong fibres of collagen up to 3 micron in diameter.
Fibroblasts - Apart from the precursors of collagen, fibroblasts also excrete elastic fibres and reticular fibres. The suffix '-blast' simply means 'active cell'. In stable conditions, these cells can switch to a less active state, in which case they are referred to as 'fibrocytes'.
fibroblasts play an essential role in wound healing. So, when tissue is damaged, fibrocytes become activated by growth factors and the resulting fibroblasts are induced to undergo mitosis (cell division) and migrate to the site of the wound.
Growth Factors - Early efforts to culture mammalian cells had only limited success. It was found that, when normal human tissue cells were cultured in standard conditions, only about 50 cell divisions would occur before they stopped dividing and died.
It was then found that, in addition to the usual nutrients - glucose, amino acids and vitamins - successful cell proliferation would only take place in a medium containing serum, the fluid derived from blood after it has clotted. It was soon shown that there are certain highly specific proteins within serum that, in very small quantities, will ensure continued cell proliferation. These proteins are called Growth Factors.
When blood clots, platelets within the clot are stimulated to release these growth factors from their secretory vesicles. One of the first to be identified was platelet-derived growth factor, or PDGF. We now know of about 50 growth factors. Within this range, there are families of factors, one of which is the fibroblast growth factor (FGF) family which has at least seven members.
|
One Important Conclusions
should be Obvious from this: |
Now, I've said that fibroblasts play a vital role in wound healing.
Fibroblasts are stimulated into activity by the presence of growth factors
that are released in the early stages of this process. Without growth factors, fibrocytes cannot be stimulated to become
fibroblasts. With the prolonged presence of growth factors, fibroblasts cannot return to their
dormant condition. Also, Dupuytren's cords are much thicker than
the normal ligaments around which they form and, significantly, there are many reports in the
literature that state that, when examined under the
microscope, these 'diseased' cords look no different from normal collagenous tissue[3].
Such protein must have been created by normal fibroblast activity under the
influence of growth factors even though no wound was present. It
follows, therefore, that the root of the problem is the anomalous presence
of growth factors in the subdermal tissue.
Wound Healing - Soon after injury to the skin, a complex series of events
occur to stop the bleeding, close the wound and repair it. These events
can be divided into four phases which are Haemostasis, Inflammatory,
Proliferative and Maturation (Remodelling). The speed and degree of
overlapping of these phases will vary depending on circumstances.
The bleeding is
stopped (Haemostasis) by the enzyme thrombin acting on a soluble factor in blood called fibrinogen
to produce an insoluble monomer (the basic component of a polymer chain) called fibrin.
Fibrin then rapidly polymerizes (forms long molecular chains) to create a fibrous blood clot that seals off
any damaged blood vessels and, ultimately, forms a scab.
In the Inflammatory phase, a range of scavenger cells called
phagocytes engulf and digest bacteria, damaged cells and any potentially
infectious material. Various factors included within the blood clot
release growth factors that promote cell
proliferation, stimulate inactive cells and induce cell migration. Because
prolonged inflammation can lead to tissue damage, it is good clinical
practice to minimize this phase by keeping the wound as clean as possible
and avoiding slapdash surgical closure. This facilitates epithelial
cells proliferation under the scab enabling closure of the wound with a new
epidermal layer that excludes infection. There is good evidence to suggest that
the continued presence of macrophages (a type of phagocyte) as a result of
infection will inhibit subsequent phases [13]
which, in effect, slows the healing process.
About two to three days
after injury, the Proliferative phase starts in which an essential process
called Angiogenesis occurs. This is the creation of new blood capillaries in
developing granulation tissue. Throughout this and the following stages, it
is essential that a good blood supply is established and maintained to bring
oxygen and nutrients to the healing process. Limited hypoxia (oxygen
deficiency) promotes growth factors and
fibroblast proliferation. However,
increased hypoxia will inhibit the fibrotic component of the provisional
extracellular matrix, inhibit epithelial cell production and can lead to excessive fibrotic scarring.
Also, hypoxia produces pronounced numbness. All of these symptoms
were evident in the condition of my left hand when the splint was removed.
The Maturation phase can last for a year or more.
During this phase, the original type III collagen fibrils degrade and are
replaced by stronger Type I collagen. Also, the older disorganized
placement of the fibrils is lost as fibroblasts secrete and align the new collagen in such a
way as to oppose stresses being applied to the tissue.
Implications - This last point
is very important as it refutes one of the main arguments employed to
support the use of a splint during the period immediately following the
operation. Every surgeon I questioned, who still insisted on fitting a
splint, claimed that allowing the patient to contract their fingers would
result in permanent contracture. They maintained that, without a
splint, patients would hold their fingers closed in an instinctive move to
protect the area of the incision. Also, when sleeping, the patient's fingers would naturally relax into a semi-closed
position. There are numerous problems with these arguments.
First, during this early phase of the healing process, the collagen
being laid down is type III. It is, for the most part,
disoriented and has limited strength. Physiotherapy and normal
finger usage would easily overcome any constriction that it may
cause - I know this from personal experience with my right hand.
Dupuytren's recontracture does not occur
during the Maturation phase. The rate of recontracture
peaks three to four years after the operation. Maturation should be
substantially complete between one to two years after the operation. So, although
recontracture may well involve collagen that was deposited during the Maturation
phase, it occurs long after the completion of the healing process.
Recontracture therefore involves original
collagenous bands left by the surgeon that have become diseased (as in my
case) and/or collagen fibres deposited during the Maturation phase that
subsequently become diseased. Either way, it should be obvious that
encasing the patient's hand in a splint will certainly not prevent recontracture. Quite the contrary,
the conditions created by preventing movement of the fingers will greatly
increase the probability of rapid and permanent recontracture after the
removal of the cast. Preventing movement of the fingers inhibits blood flow and upsets the delicate
sequence and timing of the phases of the healing process. It also
prevents blood clots from being dispersed allowing growth factors to pool
and collagen deposition to penetrate deep into tissue where normally it
would not occur. For example, it is common for surgeons to find that they are
unable to release recontracture, especially if it has advanced beyond 90º.
In an investigation of seven amputated fingers severely contracted by
Dupuytren's disease, Andrew[1]
reported that extension of the PIP joints could only be achieved by
releasing ligaments that hold the joints together - in particular,
the collateral ligaments and the volar plate. Normally, these deep
ligaments are not subject to Dupuytren's disease and would not prevent full
extension during the first operation[15, P223-224]. However, there is a very high
probability that they will become affected if there is prolonged recontracture after
an operation. It would seen to be obvious that factors released during the healing
process were implicated in the spread of the disease into the deep tissue and that making every
effort to reduce trauma and expedite healing will minimize recontracture. Bearing in mind that Dupuytren's disease is caused by the
anomalous deposition and contraction of collagen, it is surely perverse to
follow an operation to remove this tissue by doing the very thing that will
encourage its proliferation. Yet, by
forcing the patient to wear a splint, that is exactly what happens.
I should also point out that two
experienced surgeons have told me that they do not use splints because they
have found that splints make no difference to the outcome. One has to ask
why it is that some surgeons ignore the advice and experience of their
knowledgeable colleagues and persist with procedures that defy science and
common sense.
Getting back to the use of the Bruner incision mentioned in
the anatomy section, another frequent justification for using this incision is that
contractile tissue forming along a straight scar will inevitably cause
recontracture. A zigzag incision, it is claimed, would allow surface
tissue to stretch. Nice idea but not true! During the Maturation
phase, type I collagen is laid through tissue that is subject to tensile
stress - i.e. tissue that is frequently stretched. In both my hands,
there is clear evidence that collagen fibres have been laid subdermally
along straight lines that run from the fingers proximally across the palms.
In other words, collagen has been laid in straight lines to oppose the
stretching of the skin caused by extending the fingers. The deposited
collagen does not follow the lines of
the incisions. This would happen no matter what the pattern of the
incision. Also, surface scar tissue does not by itself cause Dupuytren's recontracture and will, given time,
diminish as the scar matures - a process that could take many years. However, there is a possibility that
deeper developing Dupuytren's cords could invade the subdermal tissue and
attach themselves to the scar tissue. In such cases, it is the cords
that are securely terminated to, for example, the tendon sheaths that cause the recontracture, not the
dermal scar tissue.
One of the problems confronting any surgeon
attempting to release recontracted fingers is the amount of collagenous
material that will have invaded the surrounding tissue. For example,
by the time recontracture has advanced to the point where an operation is
necessary, collagen will have invaded the subcutaneous fat.
Trying to remove the cords can so damage the skin that it is no longer
viable; in which case, a skin graft would be necessary. In other
words, during the first operation, the diseased cords are clearly
identifiable and their removal is usually straightforward. Subsequent
operations to resolve recontracture are hampered by collagen that has spread
into the skin, the deep tissue and the joints. Also, the
previous surgeon could have left the neurovascular bundles displaced from
their usual positions and they could be so enveloped in scar tissue that
they are hard to identify. I shall return to the application of skin grafts as
they offer significant advantages and the failure to inform patients of
these has had serious
consequences.
Another problem is the potential development of
Sympathetic Reflex Dystrophy which manifests itself as pain and
stiffness in the joints. It is rare. It occurs in about 5% of
patients and is severe in about 1%. It isn't unique to Dupuytren's
sufferers but it can occur if there are any complications. It is
exacerbated by forced passive mobilization - in other words, aggressive
physiotherapy. If the condition is aggravated by this means, the
hand should be rested until the pain subsides when temperate mobilization
should be started.
Finally, there is another aspect of dystrophy
that brings us back to the questionable worth of splints. Every one that I
questioned who advocated the use of a splint had insisted that the fingers
should be fully extended. Yet, setting the fingers in a fully extended
position is very likely to create sympathetic dystrophy and one doesn't have
to do much reading to find statements such as this on the subject by Tubiana,
Fahrer and McCullough[11]: "The posture of the hand during post
operative immobilization must be in the anatomical position. Certainly
extension of the metacarpophalangeal joints must be avoided." Now, I
take this to mean that the hand should be in the relaxed position. It
should not be set with the fingers fully extended as was the case with my
left hand. One really does wonder if some surgeons who 'specialize' in
this field ever bother to read the literature at all.
Much has been published regarding the nature
and possible cause of Dupuytren's contracture. As stated previously, there are two quite distinct
forms of Dupuytren's tissue - the cords and the nodules.
Microscopic examination has revealed no
significant
difference between the closely aligned collagen fibrils and fibres found in
Dupuytren's cords and normally occurring collagen. In other
words, there is no evidence to indicate that Dupuytren's contracture is
caused by the denaturing or intrinsic changes in the length of
the
collagen fibrils per se[3]. Rather, the active stage
of the disease is characterized by rapid synthesis of new collagen similar
to that produced during the Proliferative and Maturation phases of the
normal healing process. This indicates that Dupuytren's contracture is caused by
a process similar to that of wound closure described earlier. However,
instead of fibroblasts returning to their dormant state (fibrocytes) and myofibroblasts undergoing apoptosis, the
anomalous continued presence of
growth factors stimulates the deposition of repeated layers of
collagen, each of which are then contracted by myofibroblast activity.
It has also been reported that
Type III collagen, which is virtually absent in normal adult palmar fascia,
is abundant in Dupuytren's tissue (10-20% in nodules and 30-40% in cords). Growth factors have also been
found. Of these, Transforming Growth Factor beta (TGF-β), basic Fibroblast
Growth Factor (bFGF) and PDGF have all been found to cause myofibroblasts
proliferation but the former has created particular interest because of its
ability to increase collagen production.
Gosset[8] and Hueston[10,11]
have described the morphology and location of nodules. Isolated
nodules adhere closely to the skin and are generally found in areas where no
fibrous fascia structure is normally located. They tend to occur in areas
where fatty tissue is thick, between interphalangeal creases or between the
IP and MP creases. Nodules also occur on the
anterior aspect (on the top) of the palmar fascia, never on its
posterior aspect (underneath it). This would indicate that factors
stimulating collagen growth are more prevalent in the subdermal fatty
tissue.
It is now generally accepted that fibrous
cords and nodules are two distinct forms of the disease that have developed in
different tissues. The fibroblasts that create the fibrils of
collagen have the ability to lay them alongside and bond them to extant
bands of collagen, such as the pretendinous bands etc. Stimulated by
growth factors, they recognize fibres of collagen and crawl along them,
tugging newly secreted fibrils behind them to form a repeating
staggered placement of successive fibrils linked by covalent bonds.
The result is a thick and very compact fibre with considerable tensile
strength. Alternatively, if the fibroblasts can find no pre-existing
strands of collagen on which to align the new fibrils (as in fatty tissue)
or if the regular staggered placement cannot be maintained, then the result
is a nodule - a weak, disorderly agglomeration of loosely compacted fibrils
interspersed with fatty cellular tissue that has been incorporated from the
surrounding connective tissue.
Whereas previously it was thought that the
body's own fibrous bands became diseased and underwent a fundamental
change, it now appears that collagen thickly deposited on these bands is the
actual source of the contracture. What stimulates this corruption of a process
that normally serves so well to heal our physical injuries is still a
subject for research. At least, I am unaware of any safe clinical
treatment to stop it. However, if we cannot yet prevent this disease,
there is certainly room for improvement in the methods used to correct it. Apart from minimizing trauma during
surgery, as already discussed, there is at least one surgical procedure that has a proven success record in
reducing recontracture.
As far back as 1952, Piulachs[14], and then Gordon
in 1964[7] reported that there is no
recurrence of contracture if the local skin is replaced by a free skin graft from
elsewhere - a procedure called dermofasciectomy. One suggested reason for this is that there are fewer
fibroblasts in the area from where the graft is taken - usually, the
upper arm. However, the important point is that this fact has been
known and verified[11,15] for years, yet none of the surgeons I
consulted seemed to be aware of it. Only one surgeon expressed a
willingness to do a dermofasciectomy but that was in the context of
replacing damaged skin - not to avoid recurrence.
Every surgeon I consulted made a point of
emphasizing the poor success rate of this operation. The physiotherapy
department that refused to treat me had been told by the surgeon who
operated on my left hand that a poor success rate was unavoidable. All
the surveys of patients operated on for Dupuytren's contracture have
concluded that the success rate is very poor. Yet, it now turns out
that there is a surgical procedure that does give good results and a very
low probability of recontracture under the area of the graft. Even if
the full reasons for the prophylactic effect of skin grafts are not yet fully understood,
there is absolutely no reason for not using this procedure to reduce the appalling
rate of recontracture amongst Dupuytren's sufferers.
So, to take my left hand as an example,
contracture of the PIP joints of the third, fourth and fifth fingers could have been avoided
with a separate skin graft over the proximal phalanx of each finger. The
point of this is that, even in the likely event that the surgeon would fail to
remove all collagen fibres in these fingers that had the potential to become
diseased, contracture of their PIP joints could have been avoided by removing the source of the
growth factors that enabled myofibroblasts
to develop. As removing the pretendinous cord is fairly
straightforward and recontracture of the MP joints was unlikely, a palmar graft would probably serve little purpose, assuming
the skin wasn't compromised.
Many patients will, I am sure, be concerned
that the area from which the skin graft is taken will be scarred and
unsightly. I can only say that if I had been given the choice of
having straight fingers and such a scar or ending up as I have, then I would
have unhesitatingly chosen dermofasciectomy.
For further reading on this subject, see ref
15, Pages 186 to 203.
Also, the following link may be useful:
Dupuytren's is not a trivial affliction.
Unfortunately, many in the medical profession consider it to be a cosmetic
problem. The truth is that almost every physical activity in which I engage is, to some degree,
adversely affected by it. Everything such as household tasks,
personal hygiene, driving, any work or leisure activity will be rendered more difficult or impossible by it.
If you rely on playing a musical instrument to pay the bills, then that's
the end of your career. I used to be able to type reasonably
well - not any more. I'm typing this with two fingers. So, it is
depressing to note that almost every surgeon with whom I have spoken has, in
one way or another, asked whether I found it 'inconvenient'. One activity
rendered almost impossible by this affliction is putting on a pair of
gloves. As it happens, I use a lot of latex (surgical) gloves
and I have found that putting them on is impossible unless I use the largest
size obtainable. Even so, I can only tolerate them for short periods
because of the pain caused by the pressure of thick folds of material that
form at the finger joints. Any surgeon with this condition would have
the same experience and would, I can assure you, find it very 'inconvenient'.
Such are the problems encountered by people
with this condition that, even being warned of the poor prospects, they
would feel that they have little choice but to accept the risks. It is intolerable for victims of recontracture to then discover that the
surgeon had failed to employ a proven procedure that substantially reduced this problem and that,
as a result, they were in a far worse condition with little chance of
improvement.
Because recontracture occurs three to four
years after the operation, it is too easily dismissed as a separate issue.
Yet, as I have explained, recontracture is substantially the result of
procedures used - or not used - during the operation. If some surgeons
are, for whatever reason, not using procedures that minimize recontracture, then steps should be taken to include the
consequences of their inaction in the overall official figures for the
success rate of this operation. In other words, four year follow-up
examinations should be mandatory. At the moment, official figures
give a wholly unrealistic picture of the true state of affairs because
operations are considered to have been successful if the patient walks out
of hospital with all five fingers intact and more or less straight.
Then there is the question of cost. How much cheaper it would be, as
well as beneficial to the patient, if surgeons were motivated to get it
right first time. A more realistic approach would be to recognize the
need to include recontracture avoidance as an important and necessary part
of the operation and an assessment made to see that those aims had been met.
In that way, surgeons with a poor record in avoiding recontracture could be
identified instead of allowing them to continue, as at the moment, with total impunity.
I once asked a doctor if he knew the cause of
this condition. He
shrugged his shoulders and quoted some line from literature to the effect
that these things just happen and we are subject to such vagaries as life
will throw at us.
I'm sure he was trying to inject a little light relief into a situation where he knew
of many others who had experienced the same problems that I have just related.
At any rate, I realized that he
must be frustrated at seeing his patients end up as I did. However, if doctors
are not in a position to improve the outcome of hand surgery, surgeons most
certainly are. Unfortunately, there has developed a culture of
reliance on excuses to explain away these poor results. I have been frequently
told that even with the same patient, the same surgeon, the same technique
and the same kit, the results of these operations are not predictable.
In effect, these poor results are being shrugged off as one of those things
that just happen - an act of God. It is a fact that "bad things" do not just happen. There is no
capricious deity who, on a whim, decides that this operation will succeed
whilst another will fail. Like it or not, it is a fact that the outcome
of these operations is dependent solely on the procedures used and the
professionalism of the surgeons. Before
concluding, I realize that there remains an un-answered question. It is
obvious that the third finger of my left hand did not need to be operated
on. Anyone with a basic knowledge of hand anatomy would have known
that. So why did the surgeon bother to operate on it? Well, this
troubled me for a long time until quite recently when, as part of the
research for this web page, I contacted the BUPA hospital (now Spire
Healthcare) where both operations took place. These hospitals have a
system of fixed price contracts that they offer their patients. In
other words, the patients are told that they would pay a fixed price even if
there are complications or if further expenses are incurred. However,
I have now discovered that this is not the whole truth. It seems that
there was a scale of prices for Dupuytren’s surgery. For operations
involving one or two fingers, there was one fixed price but, for three
fingers, there was another significantly higher fixed price. Needless
to say, I was not told about this at the time, either by the hospital or the
surgeon. Thinking about it, this explains a lot. When the
surgeon first examined my hand, he suddenly perked up and said “Oh, three
fingers. I don’t often get a chance to operate on three fingers”. I
thought at the time that he looked pleased at the prospect. Now I know
why. Then there is the question of why he used three separate
incisions when one would have sufficed and been clinically preferable.
Perhaps he felt that it was more important to demonstrate that he had
operated on all three fingers rather than use just one incision which would
have been perfectly adequate to enable him to straighten all three fingers.
Whatever his motives, they did not include due regard for his duty of care to his patient. It is also worth
noting that both surgeons who operated on me worked in the same buildings and
must have known one another. Yet the successes of the first were no spur to
the second to improve his results. Rather, he seemed to resent the first for
taking his patients. When I tried to show the second surgeon my right hand,
he contemptuously brushed it aside. It seems to me that if we patients
could rely on those who operate on us to be motivated to learn from the
successes of others, then, in all probability, my left hand would not be the
abomination that it is today. Unfortunately, as the surveys and my
experience proves, this ethos is not universal. One ethos that
is universal, however, is that which strongly inhibits every member of the
medical profession from criticizing a confrère. To quote one surgeon whose
facial expression clearly indicated that he disapproved of what had been
done to my left hand: ”You can’t expect me to
criticize this surgeon.” Actually, I do expect his criticism. In
fact, it is largely because he, and the rest of his profession, can be
relied upon to stay silent that we are now in this situation. There is
self-evident truth in the axiom: No organization can
self-regulate properly if it is incapable of self-criticism.
Low invasive procedures are much less likely
to cause recontracture. The multiple joints in the hands (and feet) are
particularly vulnerable to excess collagen deposition which causes
recontracture. Every surgeon knows that the healing process creates
collagen so it is hard to understand why so few hand surgeons are prepared
to adopt procedures that limit its proliferation. I started this text by
describing the relatively successful outcome of the operation on my right
hand. As I explained, this result was due, in no small measure, to one
surgeon’s endeavours to use less invasive techniques and expedite recovery.
The results plainly show that his techniques did limit collagen deposition
and were infinitely preferable to traditional procedures still widely used
today. If your surgeon cannot explain how he proposes to limit collagen
proliferation, then look for another surgeon. If your surgeon
tries to tell you that recontracture is inevitable, look for another
surgeon. Although some contracture (10˚ or so) may well occur over a long
period of, say, ten to fifteen years, there is absolutely no inevitability
about the rapid and severe recontracture that has afflicted my left hand.
That is the result of incompetence. If your surgeon
proposes to use a separate incision for each finger, as mine did, then look
for another surgeon. That is beyond incompetence. Dermofasciectomy
is a powerful tool in the battle against Dupuytren’s recurrence. It is
well documented in books and the technical press that recurrence very rarely
occurs under a skin graft. Yet, depressingly, all of the surgeons I later
consulted seemed to be unaware of this prophylactic effect and would only
consider using it to replace damaged skin. Now, obviously, each
patient is different and the merits of using this technique have to be
assessed against other procedures when deciding what is best for the
patient. However, no useful assessment can be made if dermofasciectomy
is dismissed at the outset as a means to prevent Dupuytren's recurrence.
In cases where no skin graft is used,
immobilizing the hand with a splint will serve no useful purpose. Impeding
movement inhibits the healing process and the dispersal of blood clots that
are a prolific source of growth factors. This actually encourages the
re-growth of collagen that caused contracture in the first place. In
cases where a skin graft is used, there is a requirement to hold it in place
for the first seven days. To this end, a few surgeons have developed
simple splints incorporating a pressure pad that allows some movement of the
fingers. Under no circumstances should the fingers be set fully
extended. The natatory
ligament can transmit stress from the contracted pretendinous cord of one
finger to the adjacent finger making it partially contract. It does not
follow that the pretendinous band to that adjacent finger is in need of
excision. If a pretendinous cord is causing contracture of the MP joint of
a finger, it creates a prominent ridge leading to that finger and is easily
felt under the skin. Repeated
operations are much more difficult because of the widespread scar tissue and
there is a far greater probability that they will make matters worse,
especially if they don’t include a skin graft. It should be a surgical
priority to GET IT RIGHT FIRST TIME.
For completeness, I include the following.
These procedures apply to anyone who has not yet been treated for Dupuytren's.
Before considering surgery, you should be aware of them. I have little
information other than what can be found by searching literature and the internet.
Dupuytren's sufferers should ask their doctors for information about them as an alternative to surgery.
Needle Fasciotomy (aka: Needle
Aponeurotomy)
This procedure was developed in France and evolved
into a technique described by G. Foucher in 1999. It is normally only
performed on a contracted pretendinous cord that is clearly defined.
It involves inserting a needle under local anaesthetic and using the sharp
edges of the needle to cut the pretendinous cord. The procedure is obviously far
less invasive than surgery as the patient only suffers a few small holes
made by the needle. However, there are a few serious problems that could
arise. For example, if the needle is inserted too deeply, it could
damage or sever the flexor tendons to the finger. The surgery needed to correct
this mistake is made more complicated by the presence of the
Dupuytren's diseased tissue. Then there is the need to avoid the
neurovascular bundles. Sectioning cords in the fingers is hazardous as
the cords can displace these bundles from their usual position. Also, this technique is never
used after surgery because of unpredictable displacement of the
neurovascular bundles caused by that surgery.
Recontracture is still a problem. In one
survey[15, p129], it was reported that 54% suffered a recurrent lack of extension
after an average of 2.5 years. Of all surveyed, 11% needed
further surgical treatment. The problem is that the source of the growth
factors that caused the collagen deposition which created the initial
contracture has not been
removed. When a pretendinous cord is cut and the fingers straightened,
the cut ends of the cord will separate. Whereas fibroblasts cannot rebuild a
complete pretendinous band, it has been shown that they can bridge
remarkably large gaps with new collagen. So, if these cut ends are
allowed to drift closer together by allowing the fingers to relax to a
semi-clenched position, fibroblasts will start creating new shorter strands
of collagen between the cut ends. In the early stages of this
process, it is easy the break these microscopic fibres by simply
straightening the fingers. Trials have shown that this corrective
process needs to be done every day. During this period, short strands
of collagen are not only broken but longer strands which will eventually
form the lengthened band will be built and reinforced. The longer this
latter process continues the better so, a good compromise would be a
removable splint that was worn at night and removed during the day to
facilitate normal hand exercise. Now, this technique has been tried.
Unfortunately, there has grown up among many surgeons the conviction that
this method is unworkable because the patients stop using the splints.
All I can say is that I'm not surprised. I once saw an example of
those splints. It was no more than a strip of wood to which was
attached a length
of elastic. It should go without saying that if
patients are presented with a crude, cumbersome and thoroughly impractical
device, they will soon recognize its inadequacies and stop using it.
What is not so easy to understand is why those responsible for this idiotic
design were not persuaded to do better.
I note in the personal reports on the
British Dupuytren's Society
web site that patients are again being given removable splints to wear at
night. I can only hope that some thought has gone into their
design and use made of modern construction methods - 3D printing for
example. Wearing a properly designed splint is no real hardship.
I designed one and wore it on my right hand every night for a year.
Every morning the fingers were straight. During the day, those same fingers would contract slightly.
As the year passed, this recurrence reduced. It's a slow process.
This form of collagen is produced and laid down very slowly but the results
are long term. Unfortunately, I could not make a similar splint
for my left hand. The trauma and scarring were so extensive and long
term that I couldn't make a splint that was not intolerable to wear.
When considering this method, remember this: Trauma is virtually zero
and recovery is very quick. This method never rendered a hand inoperable,
like my left hand. Some people point to a few mistakes made by
practitioners who cut so deep that they severed a tendon. I have to
say that such grovelling ineptitude is very rare. Also,
remember this, such cases are vastly outnumbered by cases like mine.
If surgery regularly produces deformed and inoperable results like my left
hand, then why has it taken over 170 years for Needle
Aponeurotomy to be reconsidered as the default treatment for this
condition?
Injection Therapy
Proteins, like collagen, have enzymes that
degrade them. They are usually given names that end in "ase", so the
enzymes that degrades collagen are called "collagenases". They attack
the matrix of the collagen fibres at selected points and destroy its
integrity. It follows that, if collagenase is injected into a diseased
cord, it will degrade and break under pressure to extend the finger.
Although this does work, it can be very painful. Here again, a search
of the internet is recommended.
Radiation Therapy
This is a promising technique that has the potential to slow the progress of
the disease if used in its early stages. Like Needle Fasciotomy, most
British patients remain ill-informed about these alternatives to surgery,
all of which were pioneered in France and Germany. It's heartening to
see web pages appearing from those who have sought out and been treated by these new and far less
traumatic
procedures.
I hope the information in this web site will
encourage and equip patients to ask searching questions and enabled them to
make informed decisions.
In the words of
Enrico Fermi: "Ignorance is never better than knowledge".
R. Ashby
I intend to publish periodic updates.
Links:
References: 1. Andrew J.G.; Contracture of the
proximal
interphalangeal joint in Dupuytren's disease.; Journal of Hand Surgery
1991:16B:p446-448. 2. Braune W.A., Trübiger
A.; Die Venen der menschlichen hand.; (1873) Leipzig. from: Grapow M.; Die
Anatomie und Physiologische Bedeutung der Palmaraponeurose.; Arch Anat Physiol.;
1887:143:2-3. 3. Brickley-Parsons D., Glimcher M.J., Smith
R.J., et al; Biochemical changes in the collagen of the palmar fascia in
patients with Dupuytren's disease.; Journal of Bone and Joint Surgery Am.;
June 1981:63-A:787-797. A
free copy is available from www.jbjs.org 4. Bruner J.M.; Incisions for plastic and
reconstructive (non-septic) surgery of the hand.; British Journal of Plastic
Surgery; 1951:4:48. 5. Conway H., Stark R.B.; Arterial
vascularization of the soft tissue of the hand.; Journal of Bone and Joint
Surgery Am.; 1954:36A:1238 to 1240. 6. Gabbiani G., Majno G.; Dupuytren's
Contracture: Fibroblast Contraction? An Ultrastructural Study;
American Journal of Pathology; January 1972:Vol 66:131-146. 7. Gordon, S.; Dupuytren's
contracture, the use of free skin grafts in treatment.; Transactions of the
Third International Congress of Plastic Surgery; ed. Broadbent, T.R.;
Washington: Excerpta Medica Foundation; (1964):page 963. 8. Gosset J.;
Dupuytren's disease and the anatomy of the palmodigital aponeuroses.; In
Reference 10(1985): pages 13-26. 9. Grayson J., The cutaneous ligaments of
the digits; Journal of Anatomy; 1940:75:164 10. Hueston J.T.;
Comment on 'Anatomy and pathogenesis of the digital cords and nodules'.; Hand
Clinics; 1991:7:659-660 11. Hueston J.T., Tubiana R.; Dupuytren's
Disease; Printed by Churchill Livingstone. (ISBN 1-443-02368-9) 12. Luck J.V.; Dupuytren's contracture - a
new concept of the pathogenesis correlated with surgical management.;
Journal of Bone and Joint Surgery Am.; 1959:41-A:635-664. 13. Newton P.M. Watson J.A. et al;
Microphages Restrain Contraction of an In Vitro Wound Healing Model;
Inflammation; August 2004:Vol 28:No 4:207-214. 14. Piulachs P. Mir y Mir
L. Consideraciones sobre la enfermedad de Dupuytren.; Folia Clinica
internacional (Barcelona); 1952:2:8 15. Tubiana R., Leclercq C., Hurst
L., Badalamente M., Mackin E.; Dupuytren's Disease; Printed by Martin Dunitz.
(ISBN 1-85317-475-0) 16. Tubiana R., Thomine J-M., Makin E.;
Examination of the Hand and Wrist; Printed by Martin Dunitz. (ISBN
1-85317-544-7)
However, there remains the question of contracture.
There must be some phase of the wound
healing process in which fibroblasts exhibit the ability to contract the
collagen that they have deposited.
The development of fibrotic tissue is an essential process during the Proliferative phase
as it helps to maintain the structural integrity of the wound
and contract it. Initially, the fibrils (produced by fibroblasts) within this tissue are type
III collagen and laid haphazardly as they connect to each other and to the
edges of the wound. Some fibroblasts undergo differentiation into
myofibroblasts which have some features that are similar to smooth muscle cells (hence the
name - the prefix 'myo' denotes muscle).
Myofibroblasts then migrate to the edges of the wound where they form
linkages between the collagen fibrils and the edges of the wound. The
myofibroblasts then pull on the fibrils which contracts the wound.
Meanwhile, other fibroblasts reinforce the newly contracted wound with new
fibrils. This process can take several weeks after which
the myofibroblasts should undergo apoptosis (spontaneous cell death).
CAUSE
A BETTER WAY
Dermofasciectomy in
the management of Dupuytren's disease. J.R. Armstrong, J.S. Logan,
et al.
COMMENT
CONCLUSION
ALTERNATIVE PROCEDURES
Historical Note: in 1834, the Baron Guillaume
Dupuytren publishes
in the Lancet the description of a treatment that
closely resembled Needle Aponeurotomy. The technique was tried but recurrence was soon reported to be a
problem and the switch was made to surgery. At the time, the cause of
this recurrence was not understood but, over the intervening years, our
understanding of protein has made impressive strides and we have long passed
the time when surgery should have been replaced by this more enlightened
procedure.
The following link is a good example:
http://www.pushpullsigns.com/dupuytrensradiotherapy.html
First Published: June 2010
Updated:
Left
Hand Section - Extended.
December 2010
Comment
Section - Extended.
Conclusion Section -
Extended and clarified.
Histology Section - Extended and clarified. April 2012
Conclusion Section - Extended and clarified. August 2018
The
British Dupuytren's Society is a quite new UK charity. Its web
site is helpful and informative.